
BioGeoChemistry of Tidal Flats
Microaggregates - a Structuring Element of the Wadden Sea Water Column
Microaggregates in the size range of 5 to 500 µm are important structural elements in the water column of tidal flat ecosystems like the Wadden Sea. They constitute to a great extent the suspended particulate matter (SPM) and cause the typical grey to yellowish-brown water colour in these ecosystems. Microaggregates are composed of various source particles such as living, senescent and dead planktonic and benthic algae, other organic debris of various origin, and of inorganic particles such as clay and silt. The source particles are glued together by exopolymeric substances (EPS) and transparent exopolymer particles (TEP) produced mainly by planktonic and benthic diatoms (Simon et al. 2002). Microaggregates are intensely colonized by bacteria, exhibit a huge reactive surface area and are involved in many biogeochemical processes. Due to the strong hydrodynamic forcing and recurrent resuspension events, they undergo rapid changes in their size structure and composition.
The major goals of our study are to investigate:
- the abundance, size structure and dynamics of microaggregates during tidal cycles and seasonally
- their elemental (C, N) and biochemical (amino acids, carbohydrates) composition
- the abundance, composition, growth and substrate turnover of free-living, aggregate-associated and surface-sediment-associated bacteria
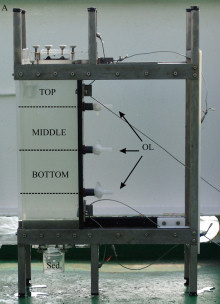
A prerequisite for these studies was the development of a suitable sampling device which allows an appropriate collection of the aggregates, the documentation of their abundance and size structure, and a reliable separation of different aggregate fractions (Fig. 1, Lunau et al. 2004).
 |
 |
| Fig 1:
New sampling device and settling chamber |
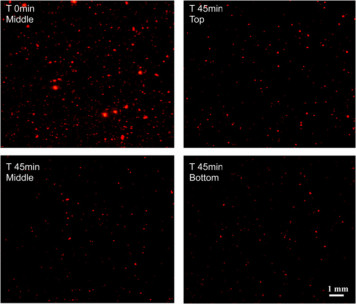
Fig 2:
Size distribution of microaggregates the initial non-separated sample (T0) and of those separated after a settling time of 45 min as the rapidly (Bottom), moderately (Middle) and slowly (Top) sinking fraction |
The newly developed device allows a simple and rapid documentation of the laser illuminated aggregates by digital photography on board ship for a subsequent PC-supported image analysis in the lab (Fig. 2). Further, the device serves as a settling chamber, allowing the separation of various aggregate fractions according to their settling behaviour (Fig. 1). The different fractions can be withdrawn by outlets (or filters) at various positions in the settling chamber for further chemical or microbiological analyses. The results show that the abundance and size structure of the microaggregates undergo rapid changes during tidal cycles with highest abundances during the current velocity maximum and largest aggregates at low (LT) and high tide (HT) (Fig. 3). We also found pronounced seasonal variations of the aggregate abundance and size, which appear to be at least partially affected by the phytoplankton (Fig. 4). Microaggregates, separated into a rapidly, moderately and slowly sinking fraction in the settling chamber after a settling time of 45 min, exhibit distinct features with respect to their elemental and biochemical composition and a rapid restructuring and microbial substrate turnover during tidal cycles.
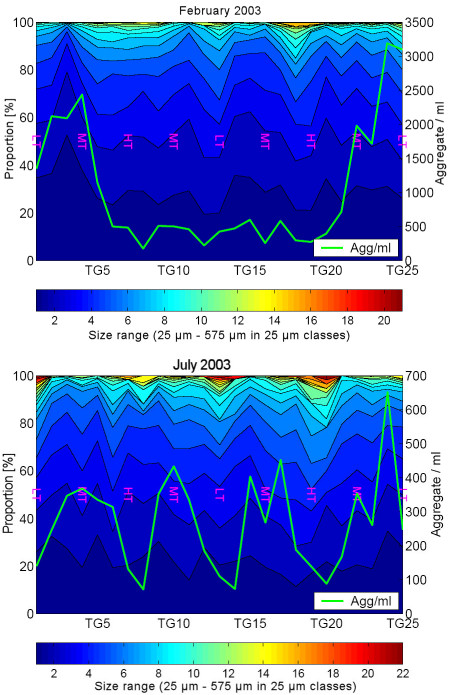
Fig. 3:
Abundance and mean diameter of microaggregates in the Wadden Sea
in the course of tidal cycles in February and July 2003
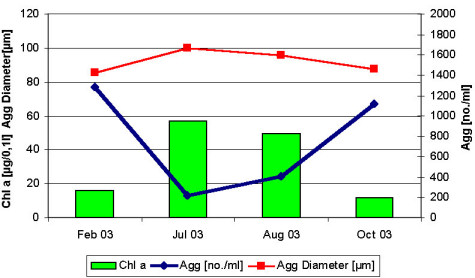
Fig. 4:
Aggregate (Agg) abundance, mean diameter and chlorophyll a concentrations (Chl a) in February, July, August and October 2003. Given are mean values of tidal cycles .
Bacterial Colonization of Microaggregates
The application of fluorescence in situ hybridisation with group-specific oligonucleotide probes showed that α- and γ-Proteobacteria and Cytophaga/Flavobacteria (Bacteroidetes) mainly colonize the microaggregates (Fig. 5). By applying denaturing gradient gel electrophoresis (DGGE) of PCR-amplified 16S rRNA gene fragments using Bacteria-specific primers and subsequent sequencing we found pronounced seasonal changes in the free-living, aggregate-associated, and surface sediment-associated bacterial communities. Each bacterial community exhibited distinct features, but the aggregate-associated bacterial community also showed overlaps to both other communities, indicating their mediating role between the water column and the sediment (Fig. 6, Stevens et al. 2005). We also found a surprisingly high diversity within the Cytophaga/Flavobacteria group during the course of the early growing season from April to June (Fig. 7), which was only detected by applying group-specific primers.
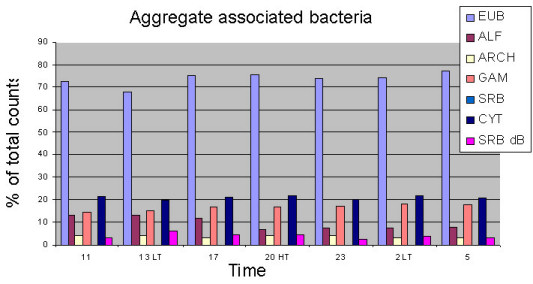
Fig. 5:
Percentages of various groups of aggregate-associated bacteria assessed by fluorescence in situ hybridisation with group-specific oligonucleotide probes during a tidal cycle in November
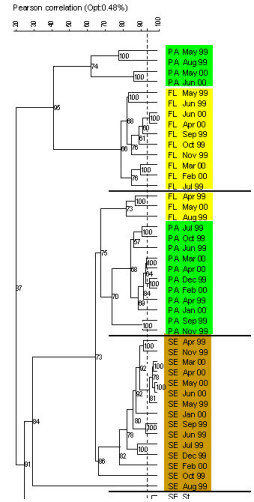
Fig. 6: Cluster analysis of DGGE banding patterns of the free-living (FL, yellow), aggregate associated (AG, green) and sediment associated (SE, olive) bacterial communities between April 1999 and June 2000 (from Stevens et al. 2005) |
DGGE Fingerprints of Aggregate-Associated Bacteria
April-June 2000 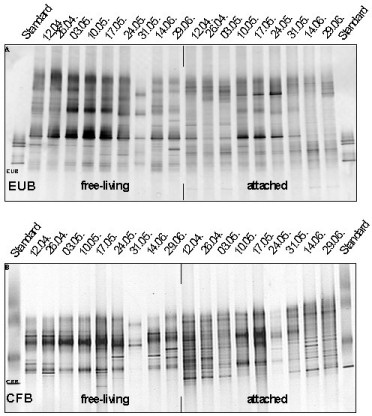
Fig. 7:
DGGE fingerprints of aggregate-associated bacteria (>5 µm fraction) assessed by Bacteria- and Cytophaga/Flavobacteria-specific primers between April and June 2000
|
In order to accurately assess numbers and dynamics of bacteria colonizing the highly autofluorescent and very abundant microaggregates in the Wadden Sea samples, we developed a new method to efficiently detach and stain bacteria associated with aggregates and sediment (Lunau et al. 2005). The bacteria are first detached from aggregates by treatment with warm methanol, followed by ultrasonication and separation of bacteria and particles by centrifugation. In a second step the bacteria are stained using the highly dsDNA-specific fluorescent dye SybrGreen I and an optically brilliant mounting medium (moviol).
Ongoing work and future perspectives
By applying the newly developed methods we have been studying intensely dynamics of the abundance, size structure and composition of microaggregates during tidal cycles and at all typical seasonal situations. Further, dynamics of the abundance, biomass production, amino acid and monosaccharide turnover of aggregate-associated and free-living bacteria are being assessed, as well as concentrations of dissolved amino acids as the major substrates of the heterotrophic bacterial communities. The composition of these bacterial communities is being analysed by DGGE of PCR-amplified 16S rRNA gene fragments using various group-specific primers (Roseobacter, Cytophaga/Flavobacteria, SAMMIC-group of γ-Proteobacteria) and by applying CARD-FISH and Micro-FISH. In fall 2004, we were able to include confocal laser scanning microscopy into our analyses of the architecture and composition of the microaggregates and of the bacterial communities using FISH and CARD-FISH.
The application of this suite of approaches and methods will greatly help to expand and deepen our insights into and presumably reveal new aspects of the role of bacteria and the significance of aggregates in biogeochemical processes in tidal flat ecosystems like the Wadden Sea.
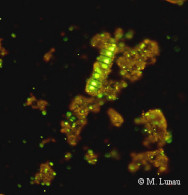 |
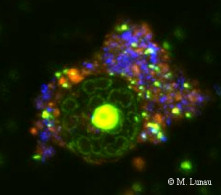 |
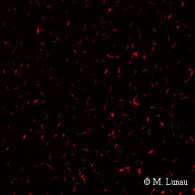
|
| Fig. 8: Epifluorescence micrograph of a SybrGreen I stained microaggregate | Fig. 9: Confocal laser scanning micrograph of a SybrGreen I stained microaggregate | Fig. 10: Epifluorescence micrograph of a culture of Muricauda sp. stained by CARD-FISH with the probe EUB338 |
People involved in this project:
Mirko Lunau, Andreas Lemke, Heike Stevens, Beate Rink, Hans-Peter Grossart, Thorsten Brinkhoff, Andrea Schlingloff, Birgit Kuerzel, Meinhard Simon, Jöran MärzRelevant publications:
- Grossart, H.P., Brinkhoff, T., Martens, T., Duerselen, C., Liebezeit, G., Simon, M. 2004. Tidal dynamics of dissolved and particulate matter and bacteria in the German Wadden Sea in spring and fall. Limnol. Oceanogr. 49: 2212-2222
- Lunau, M., A. Sommer, A., Grossart, H.P., Simon. M. 2004. A new sampling device for microaggregates in turbid aquatic systems. Limnol. Oceanogr. Methods 2: 387-397
- Lunau, M., Lemke, A., Walther, K., Martens-Habbena, W., Simon, M. 2005. An improved method for counting bacteria from sediments and turbid environments by epifluorescence microscopy. Environ. Microbiol. 7 (7): 961-968
- Simon, M., Grossart, H.P., Schweitzer, B. Ploug, H. 2002. Microbial ecology of organic aggregates in aquatic ecosystems. Aquat. Microb. Ecol. 28: 175-211
- Stevens, H., Brinkhoff, T., Simon, M. 2005. Composition of free-living, aggregate-associated and sediment surface-associated bacterial communities in the German Wadden Sea. Aquat. Microb. Ecol. 38: 15-3
| <<back | All publications | More details
|